Streamlyne Community Updates
Announcements
What's new?
December 11, 2025 - If you wish to get your flyer approved, you must submit it before December 16, 2025 to Paula Lugo (palugo@psm.edu)
Most recent community update
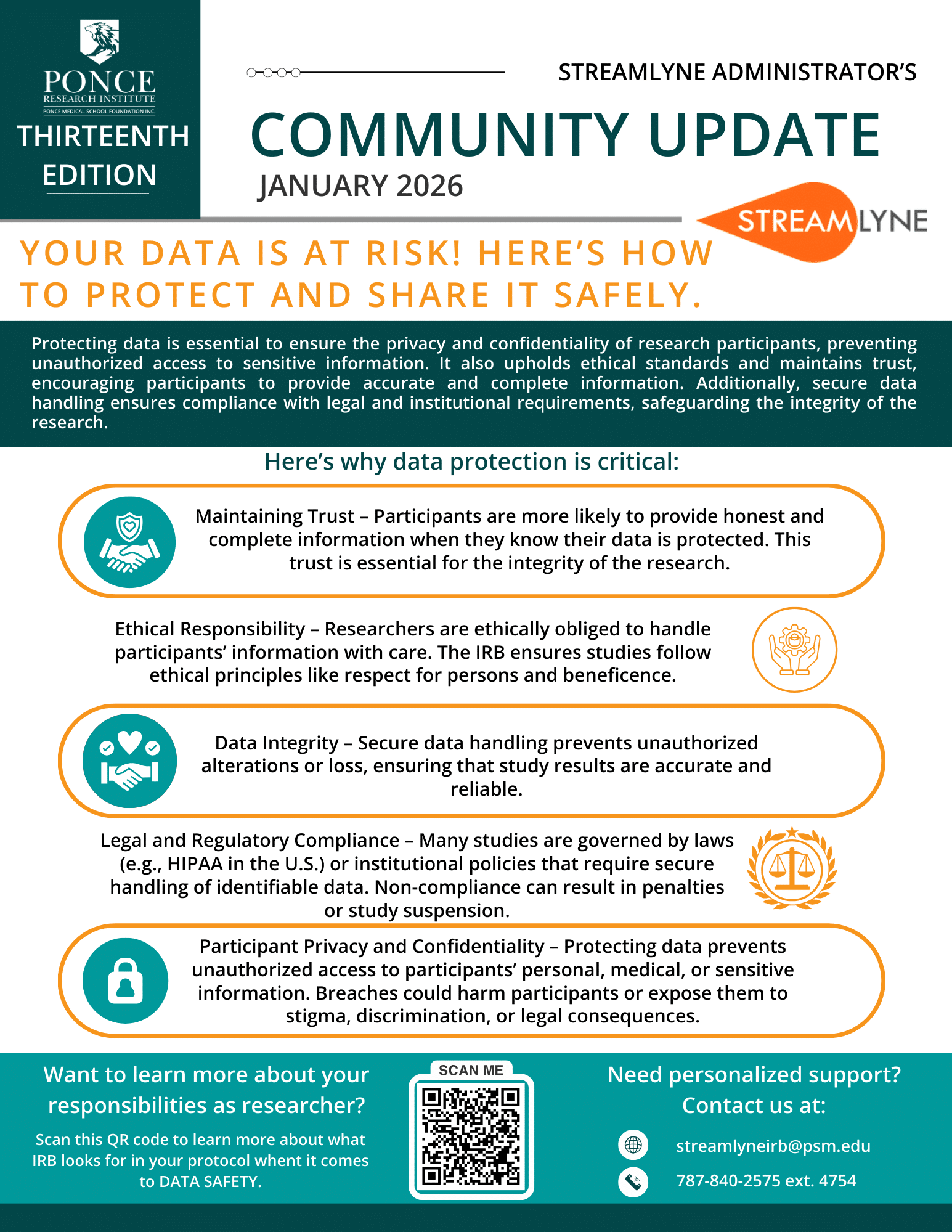
13th Edition - Your Data Is at Risk! Here’s How to Protect and Share It Safely
In this edition we will cover:
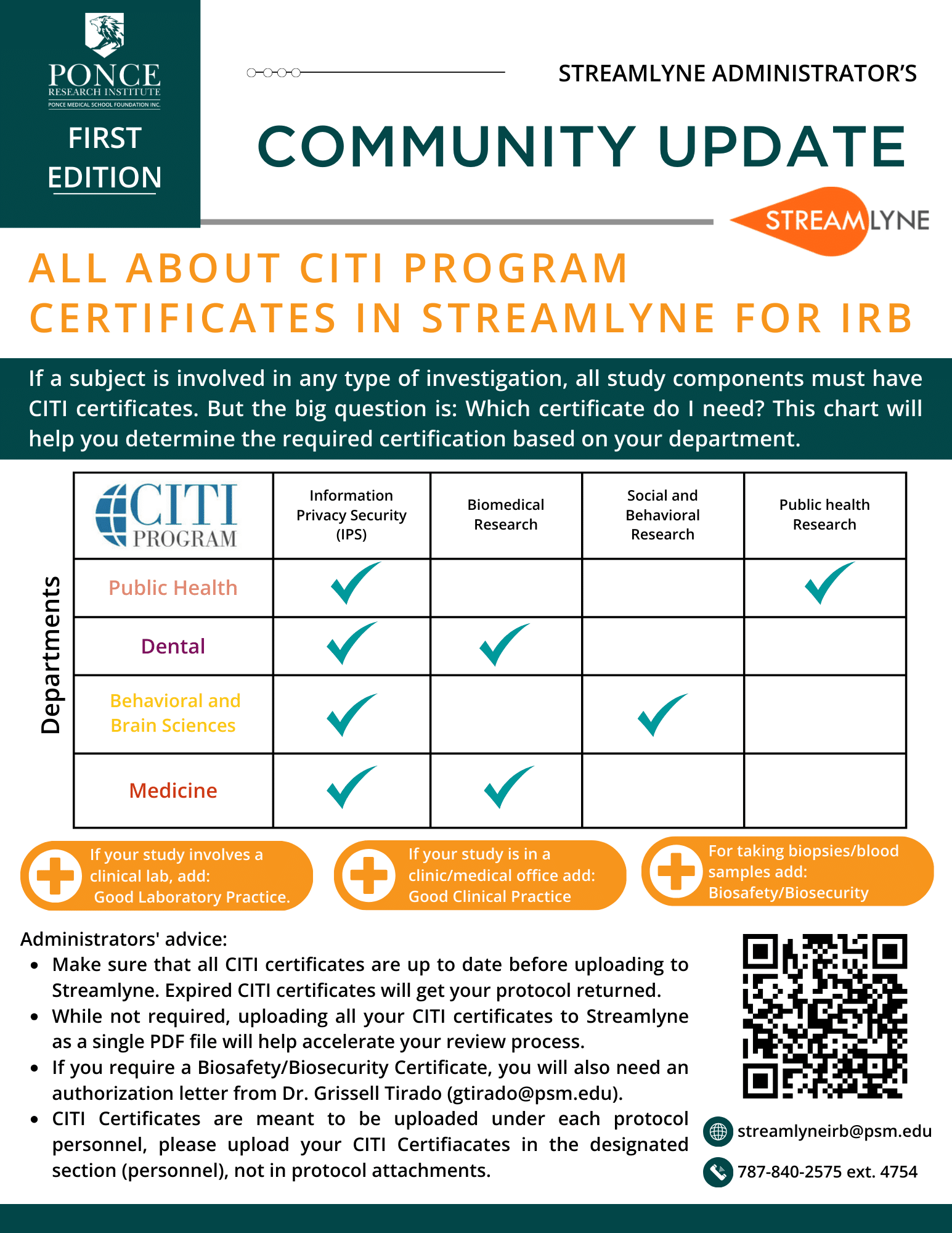
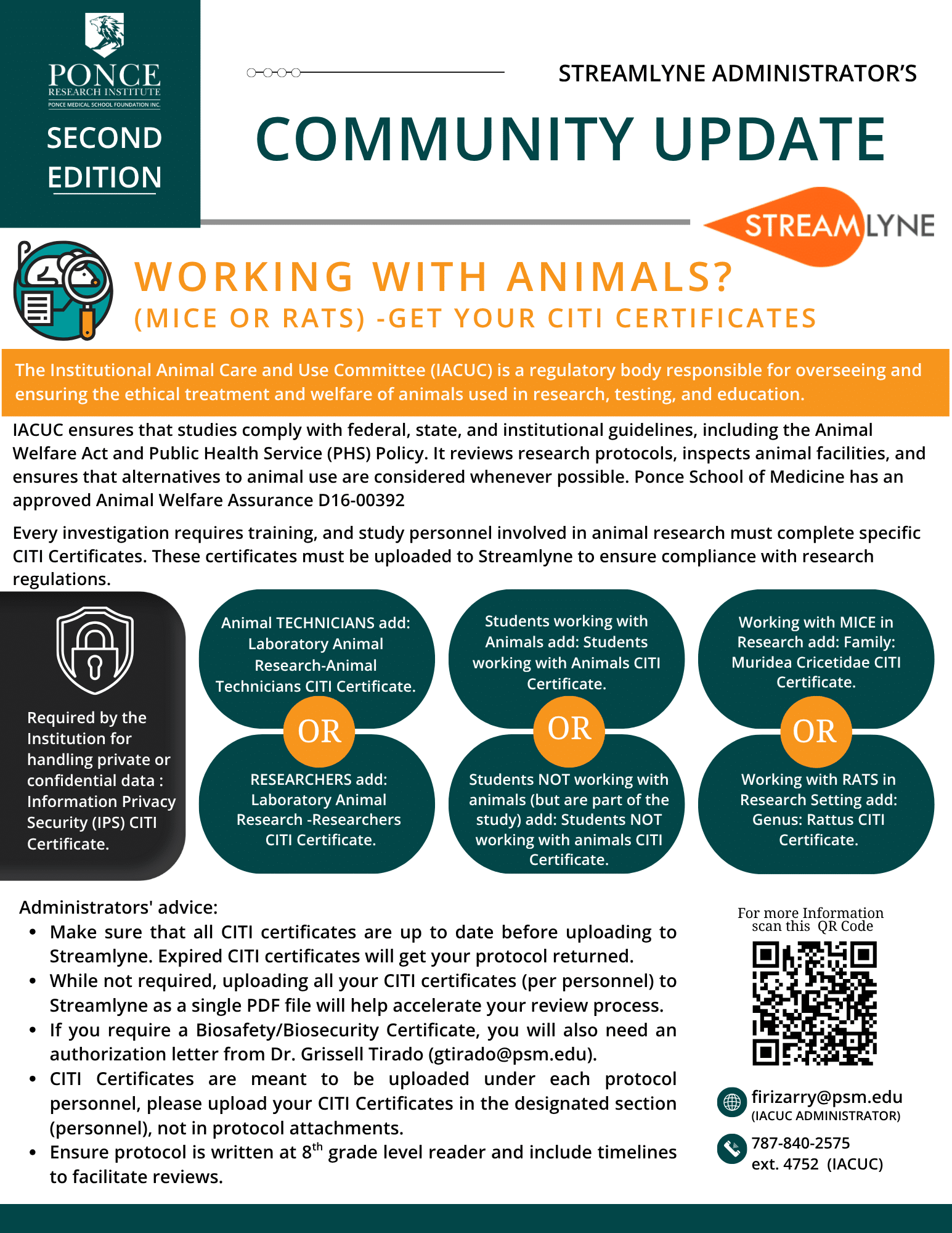
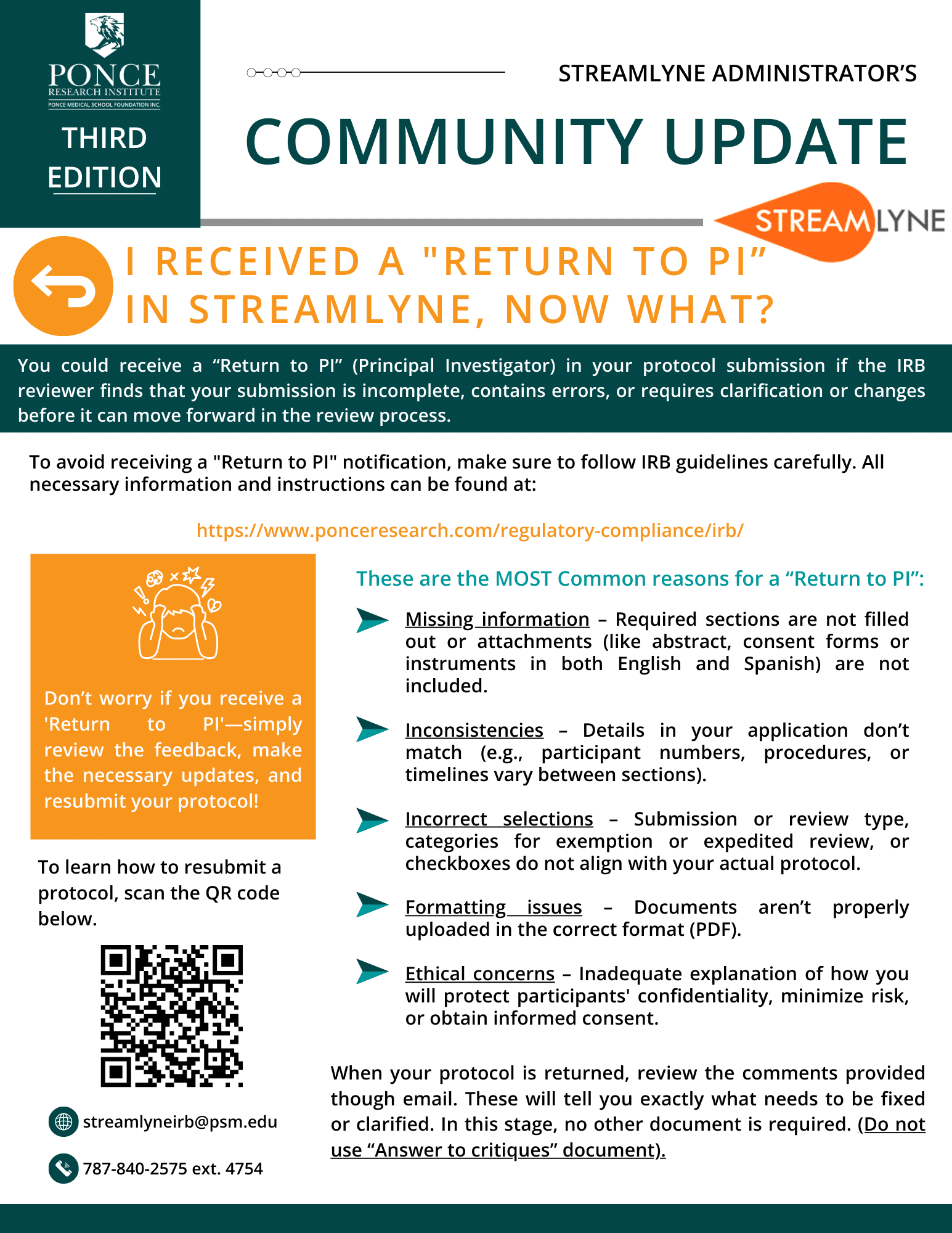
- + What is the difference between a Case Report, Case Study and Record Review
- + What documents investigators must upload to their protocol.
To view a more in depth presentation, please click here.
About Streamlyne Community Updates
The Streamlyne Community project was made for the sole purpose of helping our investigators and students familiarize themselves with the Streamlyne platform by providing them with informative flyers and presentations. Besides Streamlyne related content, we will also post any important information regarding the IRB.
When will new Community Updates be released?
New flyers with presentations will be posted monthly. Streamlyne Community Updates will first be posted as a community message on Outlook and then will be posted here the week after!
What if I can't find the subject I'm looking for?
If you are looking for a subject that we haven't covered, you can contact us via email: streamlyneirb@psm.edu and we will be happy to help you! If you would like us to cover a subject, please send us an email and it may be covered on our next edition!
Community Updates
Here you will find a list of all the informative flyers we have shared with the community. If you wish to know more, please scan the QR codes on each flyer and you will see a more detailed presentation of the subject.